Your product's flow is inconsistent, causing quality issues and wasting money. You know viscosity is key, but the science seems complex, making it hard to control your process effectively.
Liquid viscosity is a measure of a fluid's resistance to flow, often described as its "thickness." It is determined by internal friction between molecules. Understanding it is crucial for controlling how liquids behave in production, from paints and oils to foods and cosmetics.
As a factory owner producing viscometers, I talk about viscosity every day. My customers, like Jacky from Italy, are distributors who need to explain these concepts to their own clients. They know that mastering viscosity is the key to creating better products. Whether you work with lubricants, food, or cosmetics, this property affects everything from production efficiency to final quality. In this guide, I will break down everything you need to know about liquid viscosity in simple terms. Let's get started.
What Exactly Does Liquid Viscosity Mean?
You hear "viscosity" and "density" used together, but you're not sure of the difference. This confusion can lead to mistakes when you are trying to describe or modify your product.
Viscosity is a liquid's resistance to flowing, or its internal friction. It is not the same as density, which is mass per unit of volume. A liquid can be dense but not very viscous, like mercury.
Understanding the basic concept of viscosity is the first step. It is the property that makes honey slow to pour and water quick to splash. In my business, we focus on measuring this property precisely because it is so critical for industry. Let's break down the key ideas.
1.1 The Definition and Concept of Viscosity
Viscosity is a measure of a fluid's internal friction. Think of it as a fluid's resistance to changing its shape or moving. When liquid molecules slide past each other, they create friction. High viscosity means high friction, so the liquid flows slowly. Low viscosity means low friction, so it flows easily. This is why I always tell my clients that you are not just selling a product; you are selling a specific flow behavior, and viscosity is how you measure that behavior.
1.2 The Difference Between Viscosity and Density
People often confuse viscosity with density, but they are very different.
| Property | Definition | Example |
|---|---|---|
| Viscosity | A liquid's resistance to flow. | Honey is highly viscous. |
| Density | A liquid's mass per unit of volume. | Mercury is very dense. |
A simple example is vegetable oil and water. The oil is more viscous (it flows slower), but it is less dense (it floats on top of water). Understanding this difference is crucial for proper formulation and quality control.
1.3 Everyday Examples of Liquid Viscosity
You see viscosity everywhere. Water has a low viscosity, around 1 centipoise (cP) at room temperature. Honey is much more viscous, often thousands of times more than water. Ketchup, paint, and engine oil are other great examples. Their specific viscosity is designed for their function—ketchup needs to stick to food, and engine oil needs to coat engine parts effectively.
How Do Liquid Particles Affect Viscosity?
Your liquid's viscosity changes, but you don't know why. Not understanding the role of particles can make it impossible to diagnose issues or improve your formulation.
A liquid's viscosity is directly tied to its molecular structure and particles. Stronger intermolecular forces, larger particles, and the presence of additives or impurities all increase the internal friction, making the liquid flow more slowly.

When I discuss viscometer specifications with distributors, the conversation often turns to the why behind the numbers. The answer is almost always at the microscopic level. The particles, molecules, and other things floating in your liquid are in charge.
2.1 The Role of Molecular Structure in Viscosity
The shape and interaction of molecules are the main drivers of viscosity. Liquids with long, complex molecules, like polymers, tend to get tangled up. This makes it harder for them to move past each other, resulting in higher viscosity. In contrast, liquids with small, simple molecules, like water, have lower viscosity. Also, strong intermolecular forces, like hydrogen bonds, act like tiny magnets pulling the molecules together, which also increases viscosity.
2.2 How Particle Size and Distribution Affect Flow
If your liquid is a suspension or colloid, like paint or milk, the particles within it play a huge role. Larger particles or a higher concentration of particles will create more obstruction to flow, increasing the viscosity. The shape of the particles also matters. Round particles might flow past each other relatively easily, while irregular or rod-shaped particles can interlock and resist flow more strongly. This is a key factor in industries dealing with emulsions and slurries.
2.3 Solids, Additives, and Impurities in Liquids
What you add to a liquid matters. Thickeners are additives designed specifically to increase viscosity. On the other hand, a solvent might be added to decrease it. Even unwanted impurities can have a big impact. A small amount of contamination can sometimes drastically change a product's flow properties, which is why precise measurement with a reliable viscometer is so important for quality control. It helps you spot problems early.
What's the Difference Between Newtonian and Non-Newtonian Liquids?
You assume all liquids behave the same way when stirred or pumped. This mistake leads to processing problems because many industrial fluids change their viscosity under stress.
Newtonian fluids have a constant viscosity regardless of the shear force applied. Non-Newtonian fluids change their viscosity when subjected to shear, becoming thinner (shear-thinning) or thicker (shear-thickening).
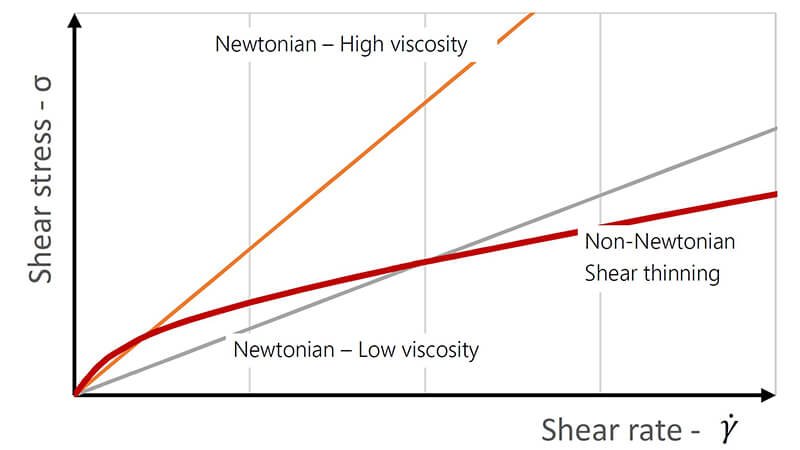
This is one of the most important concepts in rheology. I always explain this to new clients because choosing the right viscometer, like our rotational viscometers at Martests, depends on what type of fluid you are measuring. A simple viscometer might work for a Newtonian fluid, but you need a more advanced instrument to understand a non-Newtonian one.
3.1 Understanding Newtonian Fluids
A Newtonian fluid is the simplest type. Its viscosity only changes with temperature or pressure, not with the force applied to it. Water, simple oils, and solvents are common examples. If you stir water faster, it doesn't get any "thinner" or "thicker." Its resistance to flow stays the same. This makes them easy to measure and predict.
3.2 What Are Non-Newtonian Fluids?
Most complex liquids you find in industry are non-Newtonian. Their viscosity is not constant.
| Fluid Type | Behavior Under Shear | Example |
|---|---|---|
| Shear-Thinning | Viscosity decreases as shear increases. | Paint, ketchup, blood |
| Shear-Thickening | Viscosity increases as shear increases. | Cornstarch and water mixture |
Shear-thinning fluids get thinner when you stir or shake them. This is why you shake a ketchup bottle. Shear-thickening fluids do the opposite; they become more solid under impact.
3.3 Common Real-World Examples and Applications
Non-Newtonian behavior is often a feature, not a bug. Paint is designed to be shear-thinning. It flows easily when you apply it with a brush (high shear) but becomes thick on the wall (low shear) so it doesn't drip. Ketchup is the same; it stays put on your plate but flows easily when you squeeze the bottle. Understanding these properties is essential for product design.
How Can You Accurately Measure Viscosity?
You need to measure viscosity, but you are not sure which tool or unit to use. Using the wrong method can give you meaningless numbers and poor process control.
Viscosity is measured using instruments called viscometers or rheometers. The most common units are centipoise (cP) and Pascal-seconds (Pa·s). The right instrument and method depend on the fluid type and the required precision.

This is my area of expertise. At Martests, we build rotational viscometers designed for accurate and repeatable measurements. For my B2B clients, providing a reliable tool is everything. An accurate measurement is the foundation of good quality control. Let's look at the key aspects of measurement.
4.1 Common Units of Viscosity
Viscosity has specific units. The most common ones you will see are:
- Poise (P) and Centipoise (cP): The cP is very common because the viscosity of water at room temperature is about 1 cP. 1 Poise = 100 cP.
- Pascal-second (Pa·s): This is the official SI unit. 1 Pa·s = 1000 cP.
Knowing which unit your industry uses is important for communicating data clearly. My customers who resell our instruments always ask for devices that can display multiple units.
4.2 Measuring Instruments — Viscometers and Rheometers
There are many tools to measure viscosity.
- Viscometers: These are simpler instruments that typically measure viscosity under a specific flow condition. Rotational viscometers, which we manufacture, work by measuring the force (torque) needed to rotate a spindle in a liquid.
- Rheometers: These are more advanced instruments. They can measure how viscosity changes under a wide range of conditions, such as different shear rates, temperatures, and frequencies. They are great for R&D and understanding complex non-Newtonian fluids.
4.3 Laboratory and Industrial Viscosity Measurement Methods
In the lab, you would use a benchtop viscometer for high-precision quality control or research. The method often involves carefully controlling the sample temperature and following a standard procedure, like an ASTM method. In a factory, you might use an in-line viscometer that measures the fluid directly in the pipe or tank. This allows for real-time process control. Both methods aim to ensure the product stays within its target viscosity range.
What Factors Change a Liquid's Viscosity?
Your product's viscosity is inconsistent batch to batch. Not knowing what factors affect viscosity makes it impossible to troubleshoot your production process and maintain quality standards.
The main factors affecting a liquid's viscosity are temperature, pressure, and chemical composition. External forces like shear rate also have a major impact, especially on non-Newtonian fluids. Controlling these factors is key to consistency.

When a customer like Jacky calls me with a problem from one of his clients, the cause is almost always one of these factors. He sells our viscometers, but helping his customers understand why their readings are changing is a big part of his job. A good viscometer gives you the data, but you need this knowledge to interpret it.
5.1 Temperature and Pressure Influence
Temperature is the most important factor. For most liquids, viscosity decreases as temperature increases. The liquid becomes "thinner" because the molecules have more energy and can move past each other more easily. Think of how motor oil is thick when cold and much thinner when the engine is hot. Pressure has a smaller effect, but for some applications, like deep-sea hydraulics, it is very important. Higher pressure generally increases viscosity by forcing molecules closer together.
5.2 Chemical Composition and Molecular Bonds
As we discussed earlier, what the liquid is made of is fundamental. The size and shape of molecules, and the strength of the bonds between them (like hydrogen bonds), determine the baseline viscosity. A small change in the formulation, like a different supplier for a raw material, can introduce chemical differences that alter viscosity. This is why incoming material inspection is so important.
5.3 External Forces and Environmental Conditions
External forces are also critical. For non-Newtonian fluids, the shear rate (how fast you stir or pump it) changes the viscosity. Environmental conditions like humidity can also play a role, especially for water-based products where evaporation can concentrate the sample and increase its viscosity. Keeping measurement conditions consistent is essential for getting data you can trust.
Why Does Viscosity Matter for Liquid Mixing?
You are struggling to get a uniform mixture, or your mixing process uses too much energy. Ignoring viscosity can lead to poor product quality and high operational costs.
Viscosity is the most critical factor in mixing efficiency. High viscosity resists flow, making it harder to blend ingredients and requiring more powerful equipment. It determines whether the flow is smooth (laminar) or chaotic (turbulent).

Many of my clients sell to industries where large-scale mixing is a daily task. They need to control viscosity to ensure their final product is consistent. A viscometer isn't just for the lab; its data directly informs how you should design and operate your mixers.
6.1 The Role of Viscosity in Mixing Efficiency
Mixing is all about creating uniformity. If a liquid is too viscous, the mixer might only stir a small area around the impeller, leaving the rest of the tank unmixed. This is called a "cavern." It leads to an inconsistent product. If the viscosity is too low, the ingredients might not stay suspended. Getting the viscosity right ensures that you can achieve a good blend quickly and with minimal energy, which saves money.
6.2 Controlling Flow Behavior in Industrial Systems
Viscosity determines the type of flow you get.
| Flow Type | Description | Best For |
|---|---|---|
| Laminar Flow | Smooth, layered flow. Common in high-viscosity liquids. | Gentle blending of sensitive materials. |
| Turbulent Flow | Chaotic, swirling flow. Common in low-viscosity liquids. | Rapidly dispersing ingredients. |
In a pipe, high viscosity often leads to laminar flow, which is less efficient for mixing. You need enough energy to create turbulent flow to get things blended properly. Understanding this helps engineers design better pumping and mixing systems.
6.3 Design Considerations for Mixing Equipment
The mixer itself must be chosen based on viscosity. A simple propeller mixer works well for low-viscosity liquids like water. But for a thick paste or polymer, you need a specialized mixer with a high-torque motor and a different blade design, like an anchor or helical ribbon impeller. The wrong mixer will either fail to blend the material or use a huge amount of energy.
How Can You Control or Adjust Liquid Viscosity?
Your product is too thick or too thin, and you need to fix it. Without knowing how to adjust viscosity, you are stuck with off-spec batches and wasted material.
You can control liquid viscosity by changing its temperature, adding or removing components like solvents or thickeners, or by applying a specific shear rate. Each method offers a different level of control for production.

This is where knowledge becomes action. After you measure the viscosity and find it's not right, you need to know what to do. I often advise my distributors on how to help their customers with this. It turns a measurement tool into a problem-solving tool.
7.1 Increasing or Decreasing Viscosity in Practice
To increase viscosity, you can cool the liquid or add a thickening agent (a viscosity modifier). Thickeners are polymers or fine particles that create a network within the liquid to resist flow. To decrease viscosity, you can heat the liquid or add a solvent or thinner. The solvent molecules get between the larger product molecules, allowing them to slide past each other more easily.
7.2 Using Additives, Temperature, or Shear Rate
These three are your main control levers.
- Additives: Using thickeners or thinners is a direct formulation approach. This is common in the food, paint, and cosmetic industries.
- Temperature Control: Heating or cooling is a common process control method. Many mixing tanks are jacketed to allow for precise temperature management during production.
- Shear Rate Control: For shear-thinning fluids, you can make them flow more easily by pumping or stirring them faster. This is a mechanical way to manage viscosity in real time. Our rotational viscometers can help you study this effect in the lab before you scale up.
7.3 Industrial Tips for Maintaining Optimal Viscosity
In a factory setting, consistency is key. First, establish a target viscosity range for your product. Use a reliable viscometer to regularly test your batches. Monitor your key process variables, especially temperature. Also, make sure your raw materials are consistent. A small change in an incoming ingredient can have a big effect on the final product's viscosity.
Where is Viscosity Important in Real-World Applications?
You understand the theory, but you wonder how viscosity control creates value in specific industries. Knowing these applications helps you see the direct link between measurement and profit.
Viscosity is critical in nearly every industry. It determines the texture of food, the performance of lubricants, the feel of cosmetics, the application of paints, and the efficacy of pharmaceuticals.

The reason my business exists is because these applications are everywhere. Every distributor I work with, from South America to the Middle East, serves customers in these fields. They all need to measure and control viscosity to succeed.
8.1 Food and Beverage Industry Examples
In food, viscosity is texture. It's the difference between a thin syrup and a thick one, or a creamy yogurt and a watery one. We call this "mouthfeel." Companies spend a lot of time and money getting it just right because it directly impacts consumer enjoyment and perception of quality. A viscometer is an essential tool in any food R&D or QA lab.
8.2 Lubricants and Oils Performance
For lubricants, viscosity is the most important property. The "viscosity index" tells you how much the oil's viscosity will change with temperature. A good motor oil needs to be thin enough to flow when the engine is cold but thick enough to protect parts when the engine is hot. Our viscometers are used to test these oils against industry standards like ASTM.
8.3 Pharmaceuticals, Paints, and Cosmetics
In pharmaceuticals, the viscosity of an ointment determines how well it spreads, and the viscosity of a liquid medicine can affect its dosage accuracy. In cosmetics, the flow of a lotion or foundation is key to the user experience. For paints, as we've seen, viscosity controls whether the paint drips or levels smoothly. In all these cases, precise viscosity control is what separates a premium product from a cheap one.
What Are Some Common Questions About Liquid Viscosity?
You still have some basic questions about viscosity. Getting clear, simple answers to these common questions can help solidify your understanding and clear up any final confusion.
People often ask why viscosity changes with temperature, if it can be measured at home, and what the common myths are. Understanding these points makes the topic much more accessible.
I get these questions all the time, both from experienced buyers like Jacky and from technicians who are new to our instruments. Answering them helps build confidence. Here are the answers to a few of the most frequent ones.
9.1 Why Does Viscosity Change with Temperature?
This is the most common and most important question. For liquids, viscosity almost always decreases as temperature goes up. This is because heat is a form of energy. When you heat a liquid, you give its molecules more energy. This allows them to overcome the forces of attraction holding them together and move past each other more easily. The result is lower internal friction and lower viscosity. The opposite happens when you cool a liquid.
9.2 Can Viscosity Be Measured at Home?
Yes, you can do simple, non-precise viscosity comparisons at home. For example, you can see how long it takes for different liquids to flow down a tilted surface or how long it takes for a marble to fall through them. These simple experiments are great for understanding the concept. However, for industrial quality control or scientific research, you need a calibrated instrument like a viscometer to get accurate, repeatable numbers that you can compare to a standard.
9.3 What Are Common Misconceptions About Viscosity?
The biggest misconception is that viscosity and density are the same thing. As we've discussed, they are not. Another myth is that all liquids are Newtonian. In reality, most of the complex fluids we use every day are non-Newtonian. Finally, some people think of viscosity as a "bad" thing, a resistance to be overcome. But in many products, high viscosity is a critical and desirable feature that is carefully designed and controlled.
Conclusion
Controlling liquid viscosity is essential for product quality and efficiency. Understanding its principles and how to measure it accurately gives you a powerful advantage in any industry.


